Pulverized Coal Thermokinetic Properties Determination – the Drop Tube Test Facility
Coal burning plays an unsubstitutable part among primary energy resources in the Czech Republic. Especially by pulverized coal burning in power plants and heating plants we obtain a significant proportion of electricity and heat for our households and industry. However, these energy resources are out-of-date, therefore their renovation and building-up of new ones has been necessary. Modern power engineering has to meet high demands for efficiency, reliability, availability and, not least, also for minimization of impact of these large emission and waste sources operation upon our living environment. To meet these requirements, it is necessary, among others, to understand properly and to describe pulverized coal behavior during the combustion process in large-scale boilers and to make the best of these observations when designing new coal boilers.
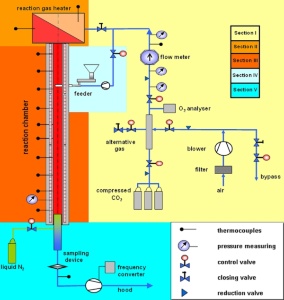
For this purpose, 1M 06059 project “Advanced Technologies and Systems for Power Engineering“ within „The Research Centres“ program of the Ministry of Education, Youth and Sports has been developed within the framework of which an experimental facility for pulverized coal thermokinetic properties determination has been built in the Energy Research Center’s testing laboratory. These properties are characterized by activation energy kinetic parameters and a pre-exponential factor and, in particular, by coal particles burnout progress. This experimental facility, the so-called drop tube, allows simulation of environment corresponding to conditions in large-scale pulverized-coal-fired boilers by setting-up temperature, oxygen concentration and reaction gas flow velocity in a reaction chamber. Gas parameters are set and controlled in a fully automatic mode.
Subsection for Nonspecialists
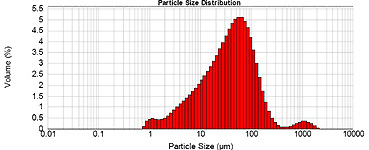
We take a small amount of pulverized coal and pour it at various height levels into the tube in which hot gas of given composition flows. Coal is dropping down and burning gradually. At the outlet from the tube, the coal and ash particles are caught on a filter and then analyzed which reveals this coal behavior when burning in a boiler.